

750 mmHg to Atm conversion calculator convert 750 mmHg to Atm and vice versa. If we want to press some kind of food in order to get a better taste, we use simple things such as stone or pile of books or just a piece of metal. Online Calculators > Conversion > 750 mmHg to Atm 750 mmHg to Atm.

There are plenty o situations which we can experience in our life. Thus, manometer is used for such situations. But for car tires more exact measurements of pressure are needed. It it easier to measure bicycle tires it with hand, without using any instruments. In ordinary life it is important to measure the pressure of tires. Air pressure is measured using special units named millimeters of mercury. As we all know, the weather is changeable, and for reason pressure changes as well. According to this factor fisherman predicts about catching fish. Climate and air pressure are related to every day activities. But this is also the case which happens not so often in daily life. Otherwise a number of various problems can occur. Pressure = 1.5 mol/ litre * 1639.For example divers, alpinists and pilots should always follow the factor. All you need to do is check the Henry's law constant in the table above, and input the numbers into the partial pressure formula: Our conversions provide a quick and easy way to convert between Pressure units. Let's say that you want to calculate the partial pressure of dinitrogen (N 2) in a container. Online calculator to convert millimeters of mercury to atmospheres (mmHg to atm) with formulas, examples, and tables. Type in your own numbers in the form to convert the units Quick conversion chart of mm Hg to atm.
#MMHG TO ATM CONVERSION CALCULATOR HOW TO#
Use this page to learn how to convert between millimeters mercury and atmospheres. Note that rounding errors may occur, so always check the results. For quick reference purposes, below is a conversion table that you can use to convert from atm to mmHg. 1 pascal is equal to 0.0075006157584566 mm Hg, or 9.8692326671601E-6 atm. Let's use the Henry's law equation in an example. Task: Convert 8 atmospheres to mmHg (show work) Formula: atm x 760 mmHg Calculations: 8 atm x 760 6,080 mmHg Result: 8 atm is equal to 6,080 mmHg. Where the mole fraction of the solute is given:.Where the concentration of the solute is given:.How to find partial pressure with Henry's law constant? There are two methods: Henry's law is only accurate at low gas pressures (pressures < 1000 hPa), constant temperatures (usually 293.15 K) and when the molecules are at equilibrium. In the table below, you can find its value for some of the most common gases in water at 298 K: Element The coefficient of this proportionality is the Henry's law constant. The partial pressure of a gas above a liquid is proportional to the amount of gas dissolved in that liquid.
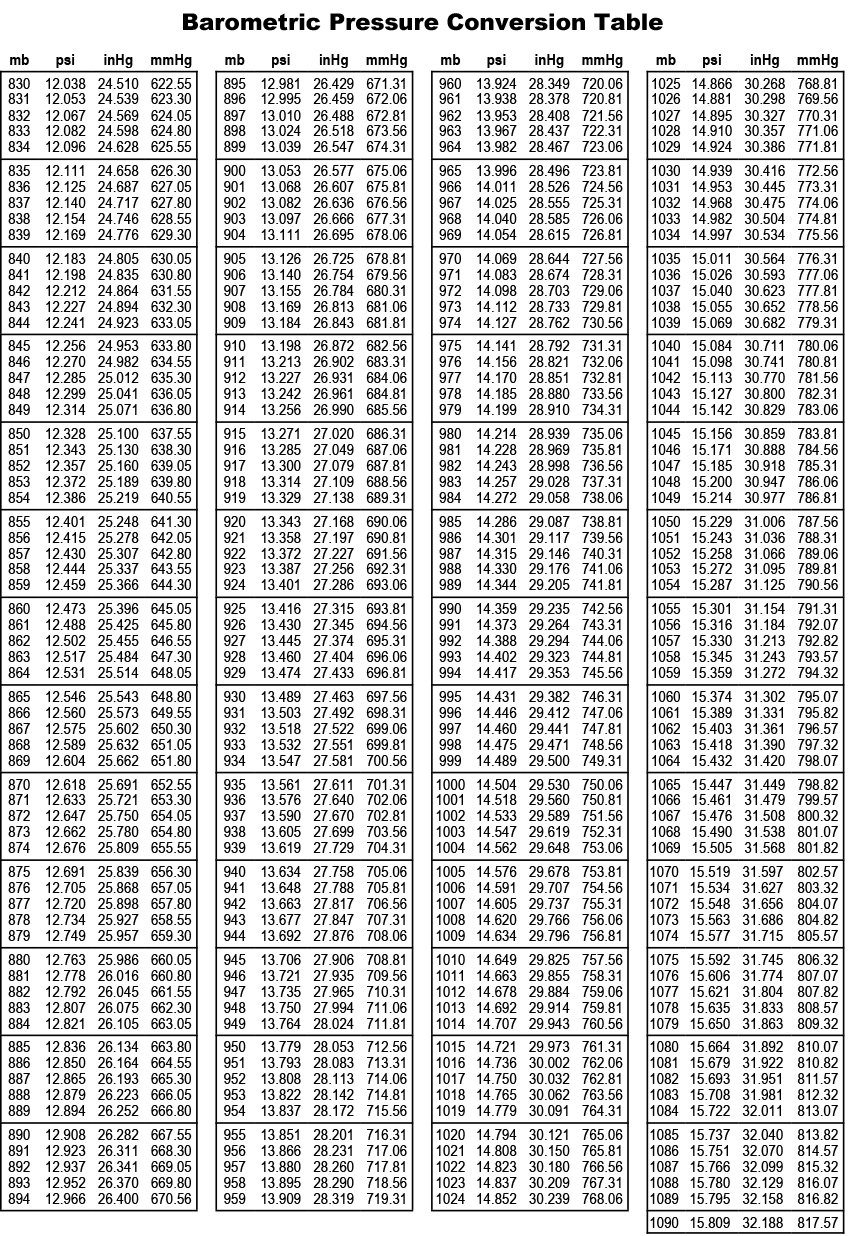
Which one you choose depends on the data you've collected beforehand. The above formula is one of our calculator's four partial pressure formulas. It shows that the partial pressure of one component is proportional to its mole fraction. Solution: multiply the atm value by 760.0 mmHg / atm. Alternatively, to find out the torr value for the most commonly converted atm value, you may check the atm to torr conversion table. 752 milliliters of mercury equals 0.989474 standard atmospheres. equals 760.0 mm Hg, so there will be a multiplication or division based on the direction of the change. To convert atm (atmosphere) to torr (mmHg) and to convert torr (mmHg) to atm, you may use the converter above. Looking at the mmHg to atm converter, you will see that we entered a value of 752 mmHg, which gives us an answer of 0.989 atm, which is rounded down from 0.989474 atm. Where mole fraction is the ratio of moles of the selected gas to the moles of the entire gas mixture. This means that to convert mmHg to atm you should multiply your figure by 0.0013157896611399. Partial pressure = total pressure * mole fraction The conversion calculation from millimetres of mercury to inches of mercury over the vacuum and atmospheric pressure range is as follows: 1 inHg 3386.39 pascals (Pa) 1 mmHg 133.322 pascals (Pa) inHg value x 3386. Where p 1, p 2, and so on, up to p n, represent the partial pressure of each gaseous component. It can also be illustrated with an equation: The total pressure exerted on a container's walls by a gas mixture is equal to the sum of the partial pressures of each separate gas. The partial pressure of one component of this mixture is the pressure that this individual gas exerts. If a mixture of ideal gases (i.e., where the molecules don't interact with each other) is sealed within a container, the gases will diffuse and fill up all of the available space.

Pressure is the force applied orthogonally over a surface.


 0 kommentar(er)
0 kommentar(er)
